POSTER ABSTRACTS
Materials should NOT be shared with those that are not registered for the conference. Poster abstracts are not proofed for spelling and/or grammar errors.
The poster and/or other information contained on this website may NOT be downloaded and/or used without prior written permission from all authors on the project. If you would like to be connected with the author(s), please email [email protected].
Integrative function of microcirculation and skeletal muscle function in peripheral artery disease
Elizabeth J. Pekas, MS1, Jonathan R. Thompson, MD2, Paras K. Mishra, PhD3, Iraklis I. Pipinos, MD2,4, TeSean K. Wooden, MS1, Cody P. Anderson, BS1, Michael F. Allen, BS1, and Song-Young Park, PhD1
1School of Health & Kinesiology, University of Nebraska at Omaha, Omaha, NE
2Department of Surgery, University of Nebraska Medical Center, Omaha, NE
3Department of Cellular & Integrative Physiology, University of Nebraska Medical Center, Omaha, NE
4Department of Surgery and Veterans Affairs Research Service, Nebraska-Western Iowa Health Care System, Omaha, NE
Abstract
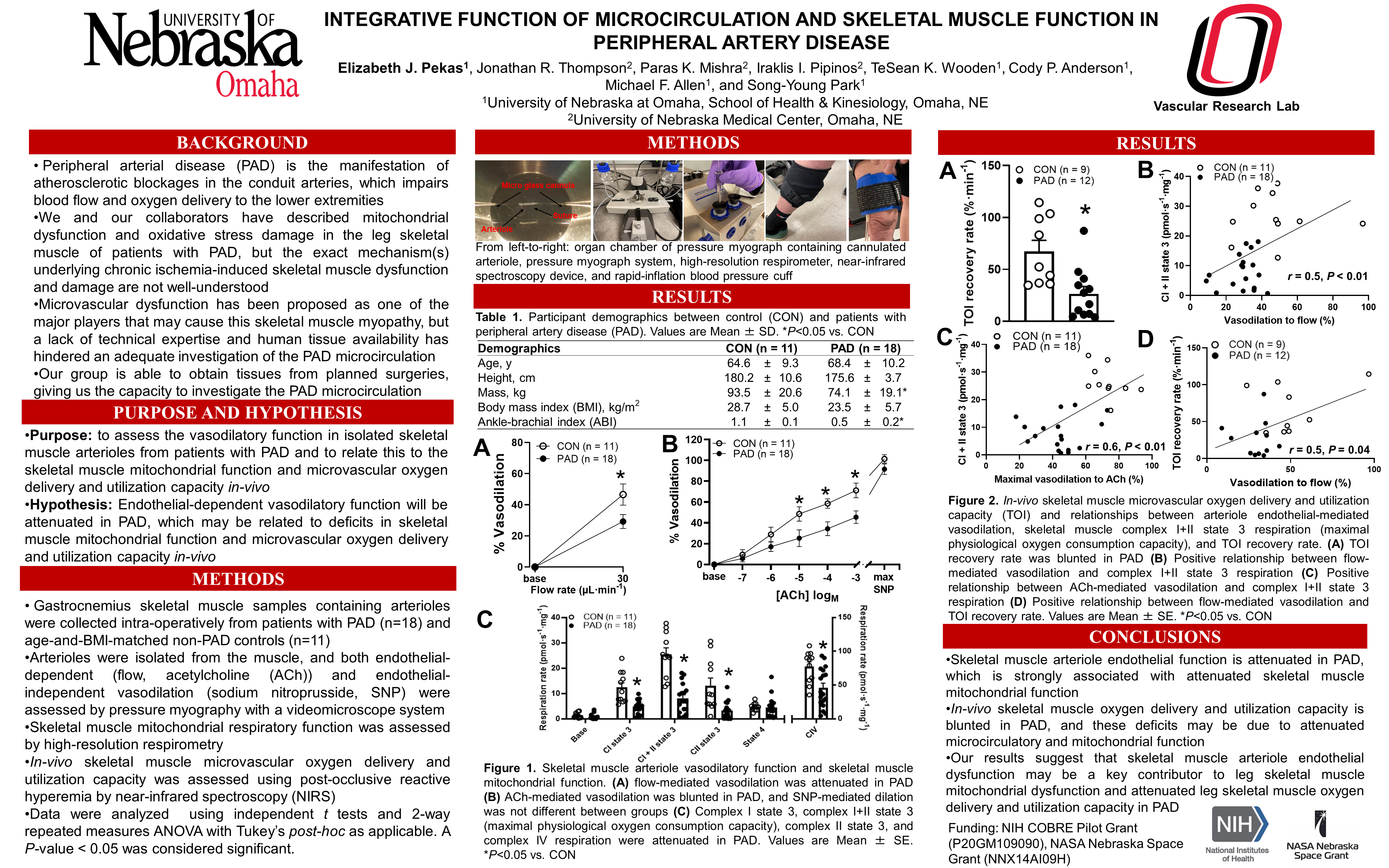
Peripheral artery disease (PAD) is an atherosclerotic disease that impairs lower-extremity circulatory function. Attenuated skeletal muscle mitochondrial function and oxygen utility capacity have been reported in the ischemic limbs; however, the underlying mechanisms are not well-understood. We investigated the impacts of chronic ischemia on skeletal muscle arteriole vasodilatory function and its contribution to skeletal muscle mitochondrial function and microvascular oxygen delivery and utilization capacity (TOI) in PAD. Skeletal muscle and arteriole samples from patients with PAD (n=18, 68.4±10.2 years) and age-matched controls (CON, n=11, 64.6±9.3 years) were harvested. Endothelial-dependent and endothelial-independent vasodilatory function was assessed by flow, acetylcholine (ACh), and sodium nitroprusside (SNP), and skeletal muscle mitochondrial function was measured by high-resolution respirometry. TOI was assessed by near-infrared spectroscopy in-vivo. Endothelial-dependent vasodilation was attenuated in PAD in response to ACh (10-3M, CON: 71.1±7%, PAD: 45.5±6%, p<0.01) and flow (CON: 46.6±6.8%, PAD: 29.2±4.5%, p<0.01), whereas endothelial-independent vasodilation was not different between groups (10-3M, CON: 101.5±4%, PAD: 91.6±5%, p=0.12). Complex I + II state 3 respiration was lower in PAD (CON: 26.1±2.1, PAD: 7.8±1.4 pmol∙s-1∙mg-1, p<0.01), and TOI was blunted in PAD (CON: 67.2±10.9, PAD: 26.6±7.0%∙min-1, p<0.01). Furthermore, flow-mediated dilation and ACh-mediated vasodilation were positively associated with complex I+II state 3 respiration (r=0.6 and r=0.5, respectively, p<0.05) and TOI (r=0.5 and r=0.6, respectively, p<0.05). These findings suggest that conduit artery atherosclerotic blockage-mediated chronic ischemia attenuates skeletal muscle microcirculatory endothelial function, which may be a key contributor to attenuated leg skeletal muscle mitochondrial function and oxygen delivery capacity in patients with PAD.
Access PDF version to expand view.